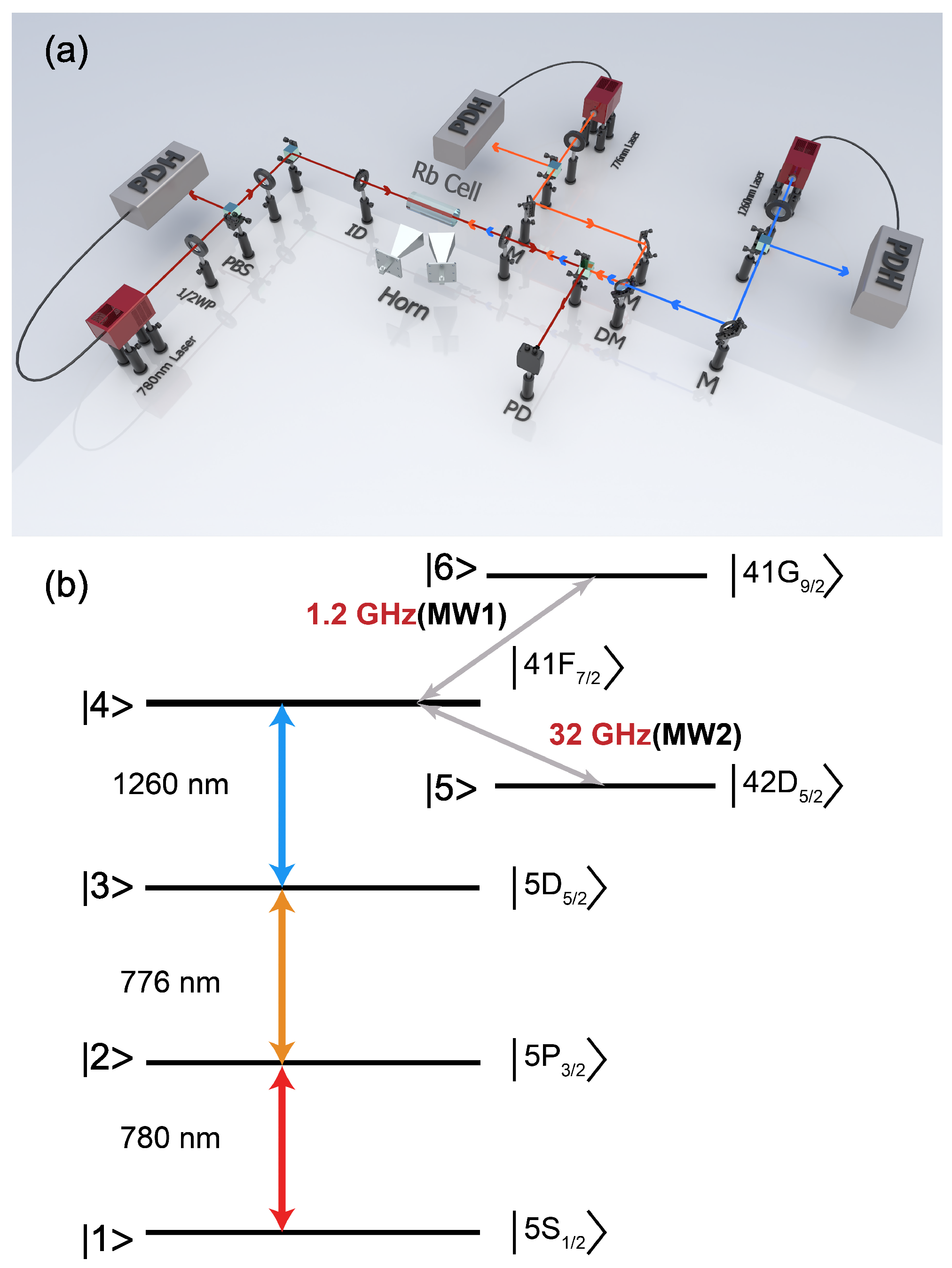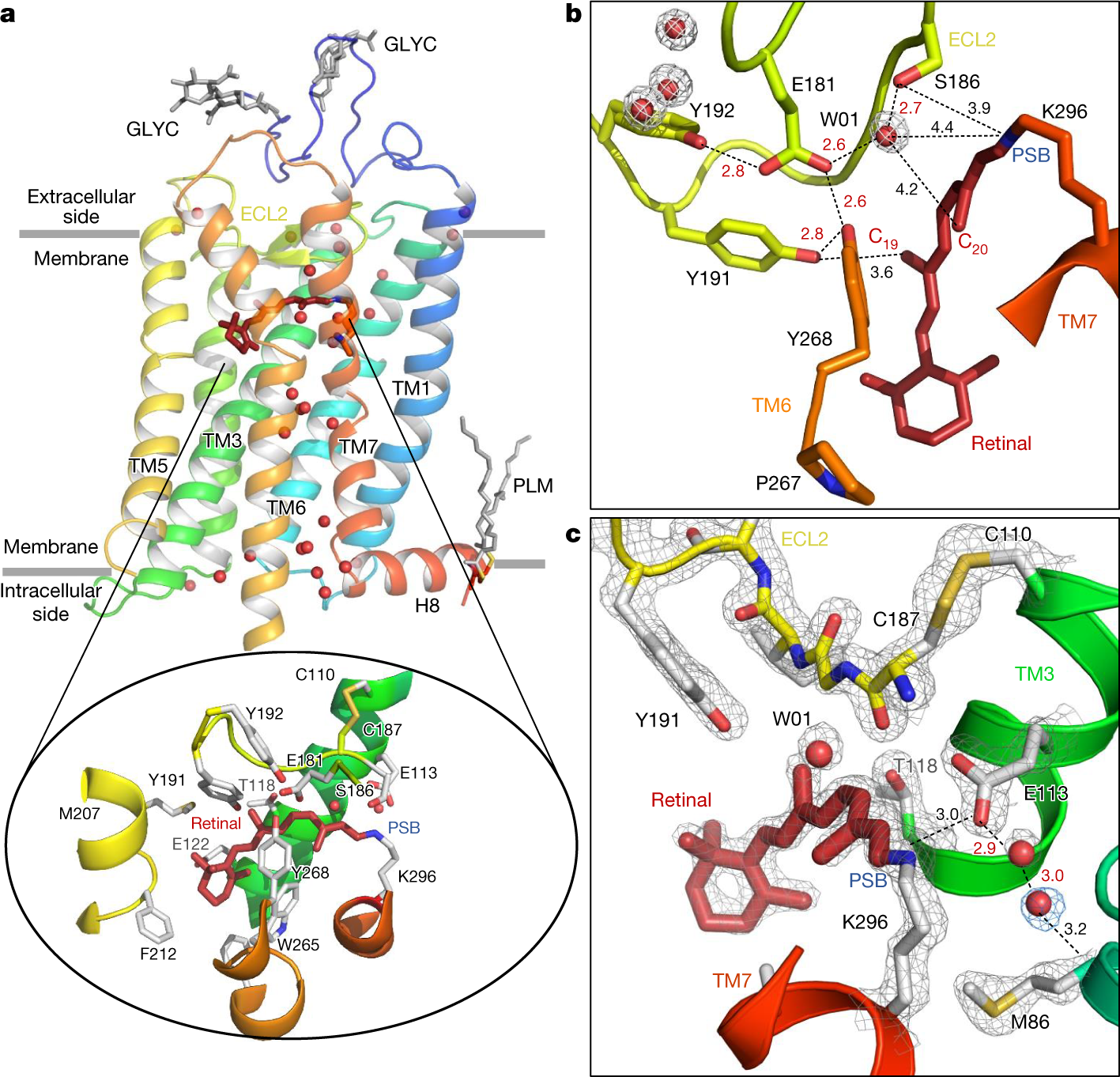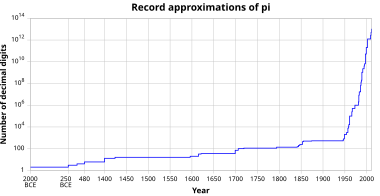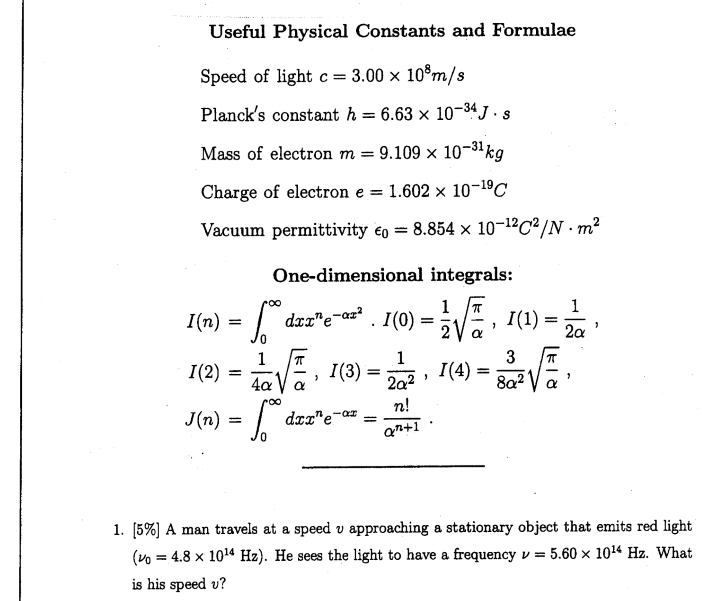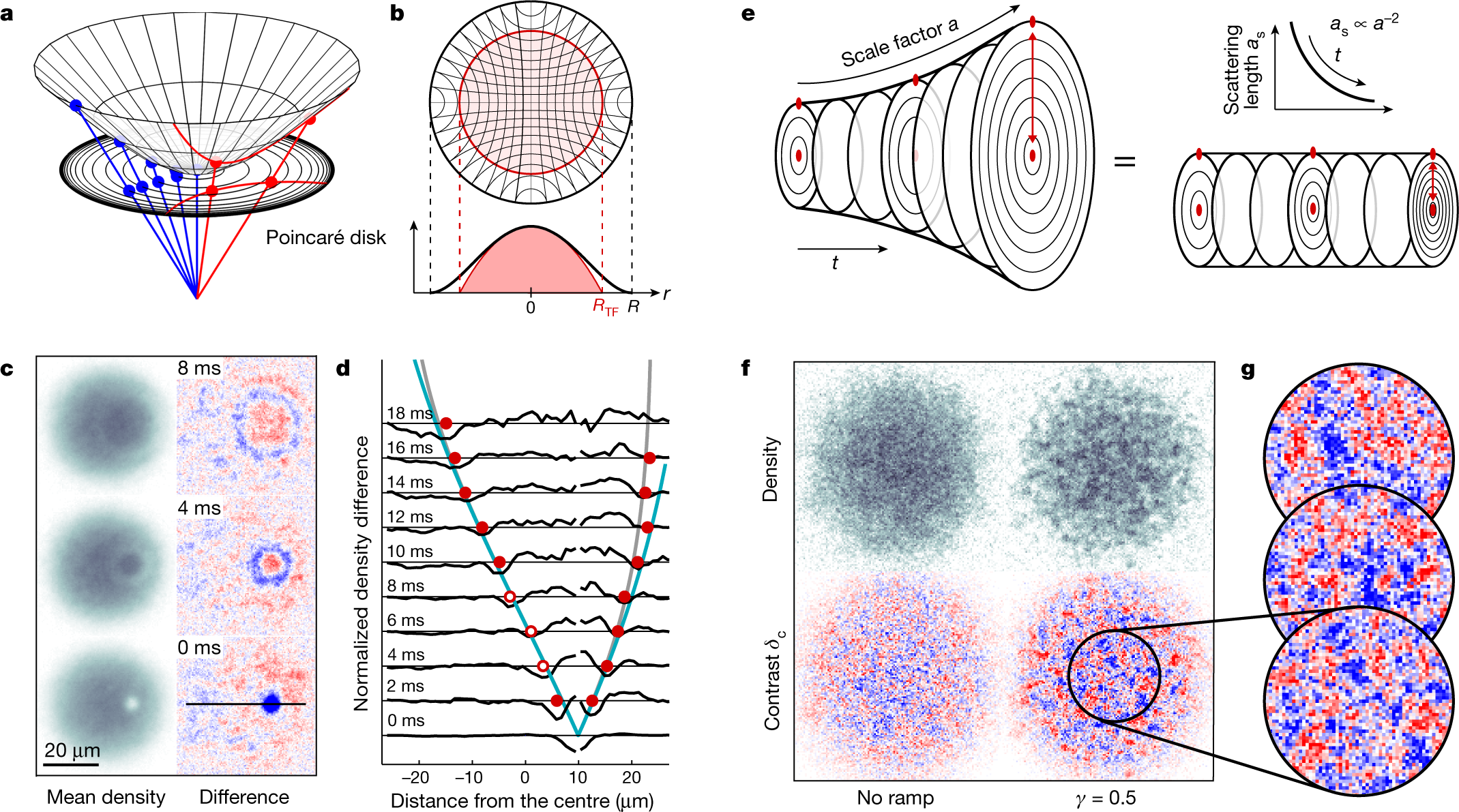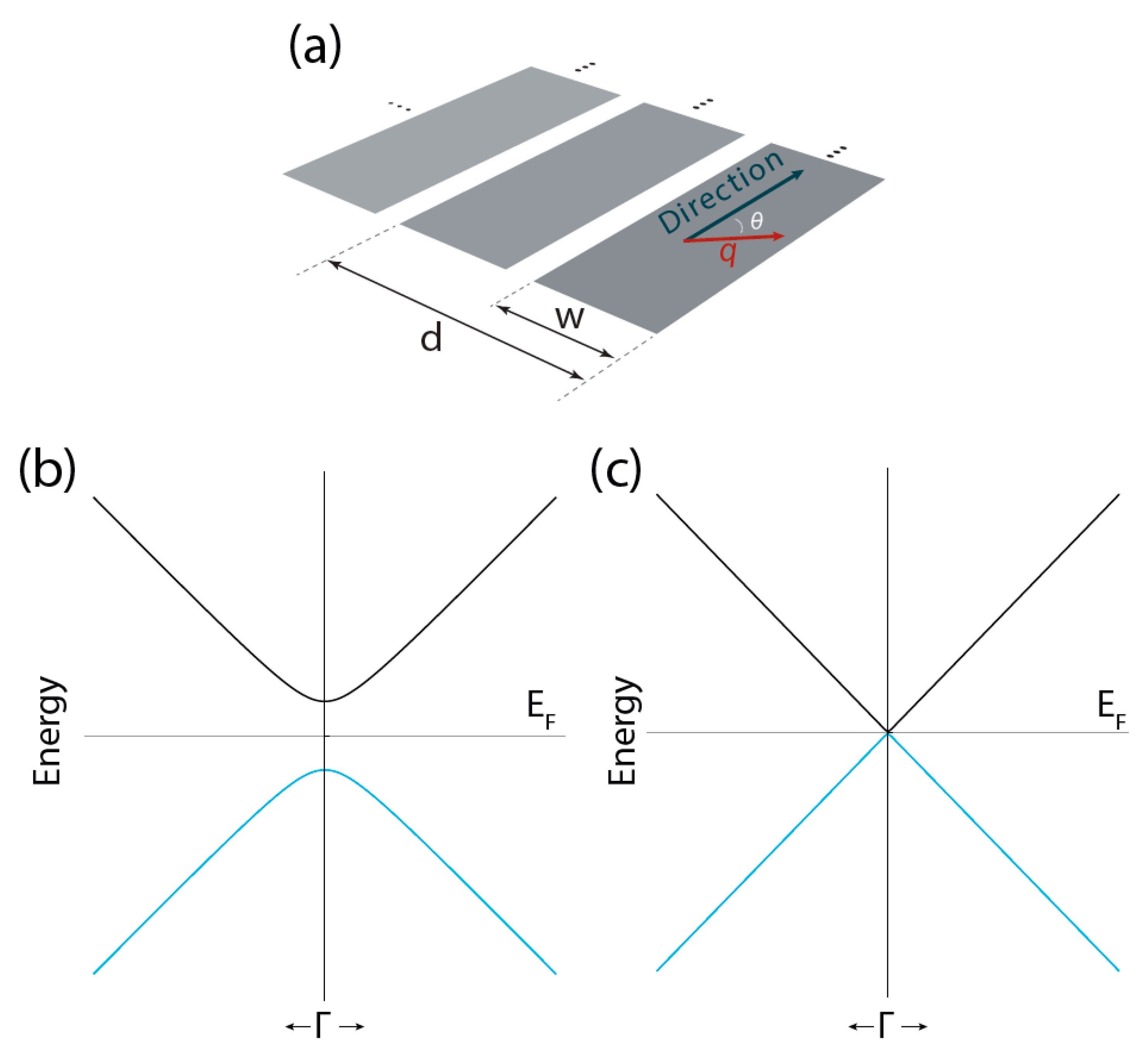
Chemosensors | Free Full-Text | Proving Surface Plasmons in Graphene Nanoribbons Organized as 2D Periodic Arrays and Potential Applications in Biosensors
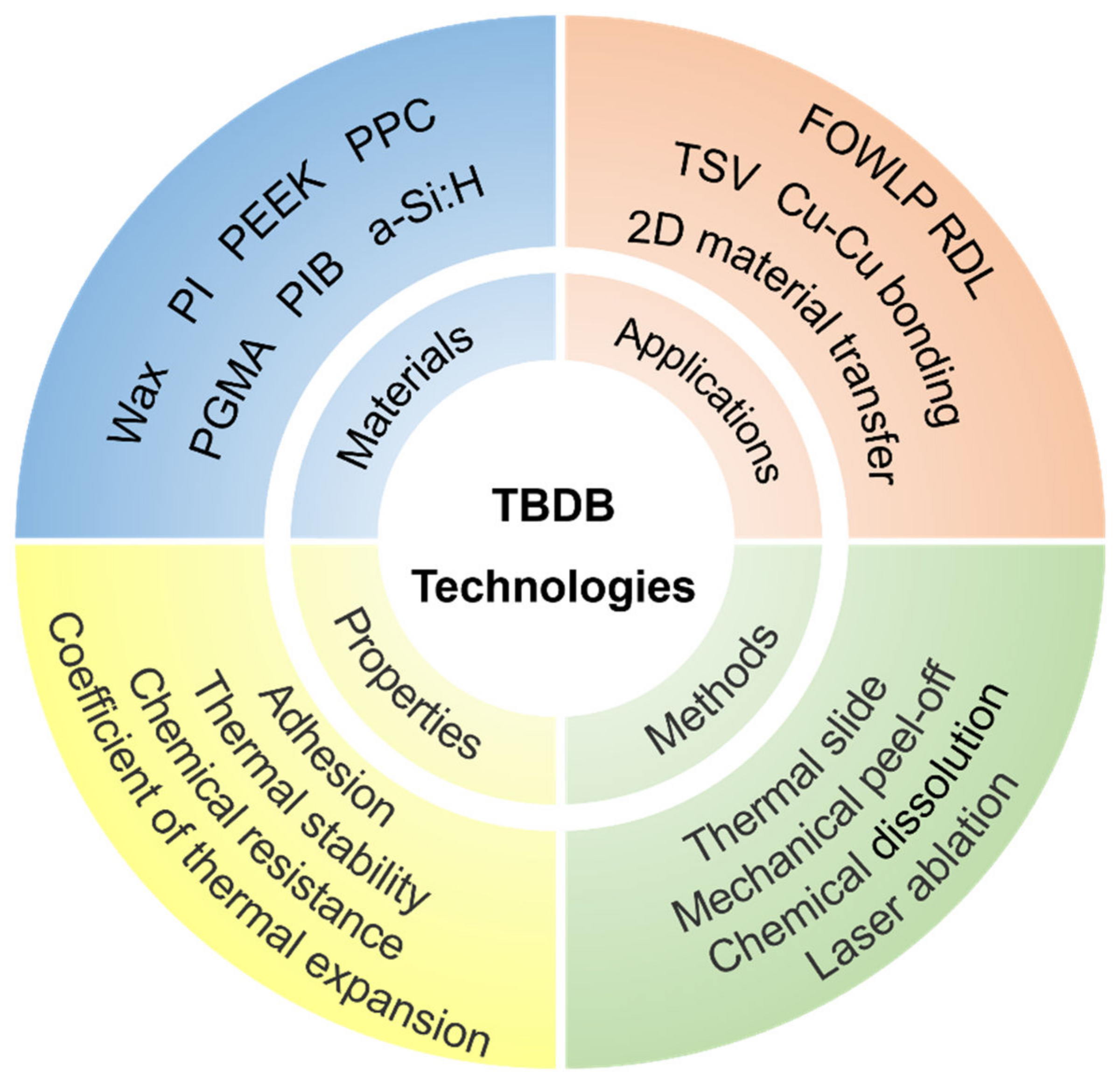
Electronics | Free Full-Text | Temporary Bonding and Debonding in Advanced Packaging: Recent Progress and Applications

Antioxidants | Free Full-Text | The Influence of 5′,8-Cyclo-2′-Deoxyguanosine on ds-DNA Charge Transfer Depends on Its Diastereomeric Form: A Theoretical Study
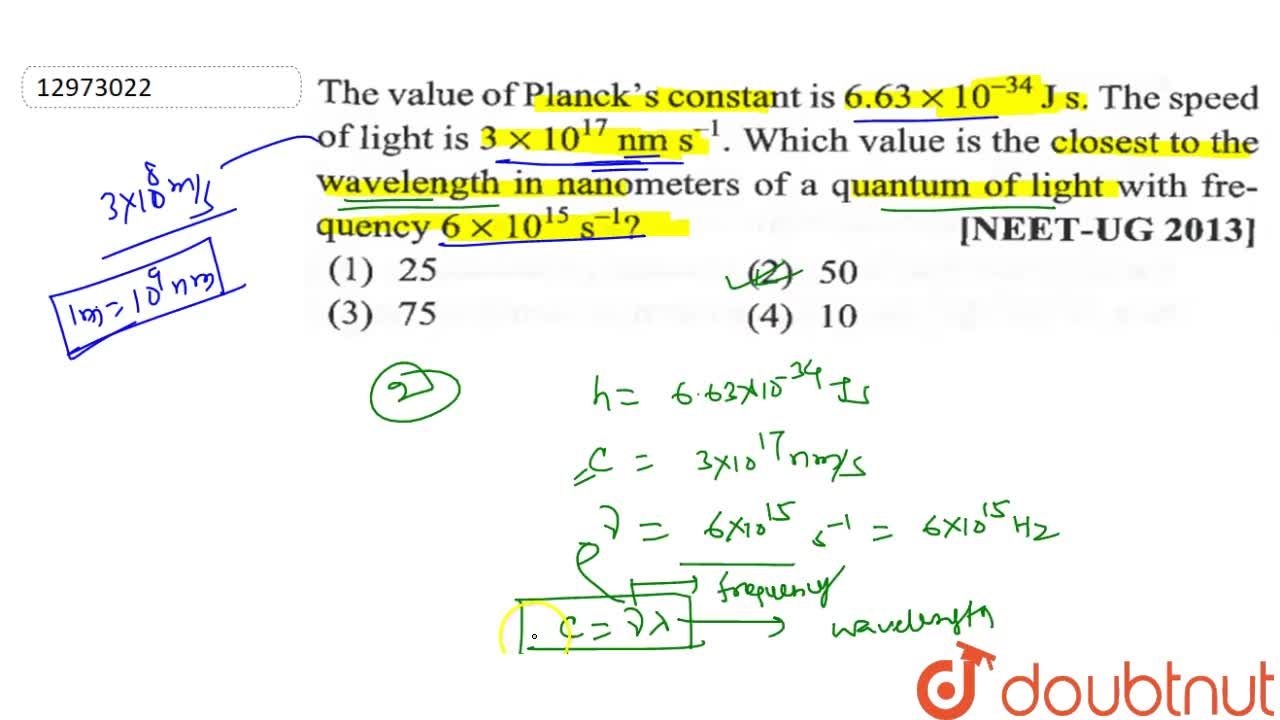
The value of Planck's constant is 6.63 xx 10^(-34)Js. The speed of light is 3xx10^(17)nm s^(-1). Which value is the closed to the wavelength in nanometers of a quantum of light with

Given: The mass of electron is 9.11 × 10^(–31)Kg Planck constant is 6.626 × 10^(–34)Js, the uncertainty involved in the measurement of velocity within a distance of 0.1Å is:-
The value of Plank's constant is 6.63 × 10 34 Js. The speed of light is 3 × 1017 nm s 1 . Which value is closest with frequency of 6 × 1015 s 1 ?A. 25B. 75C. 10D. 50
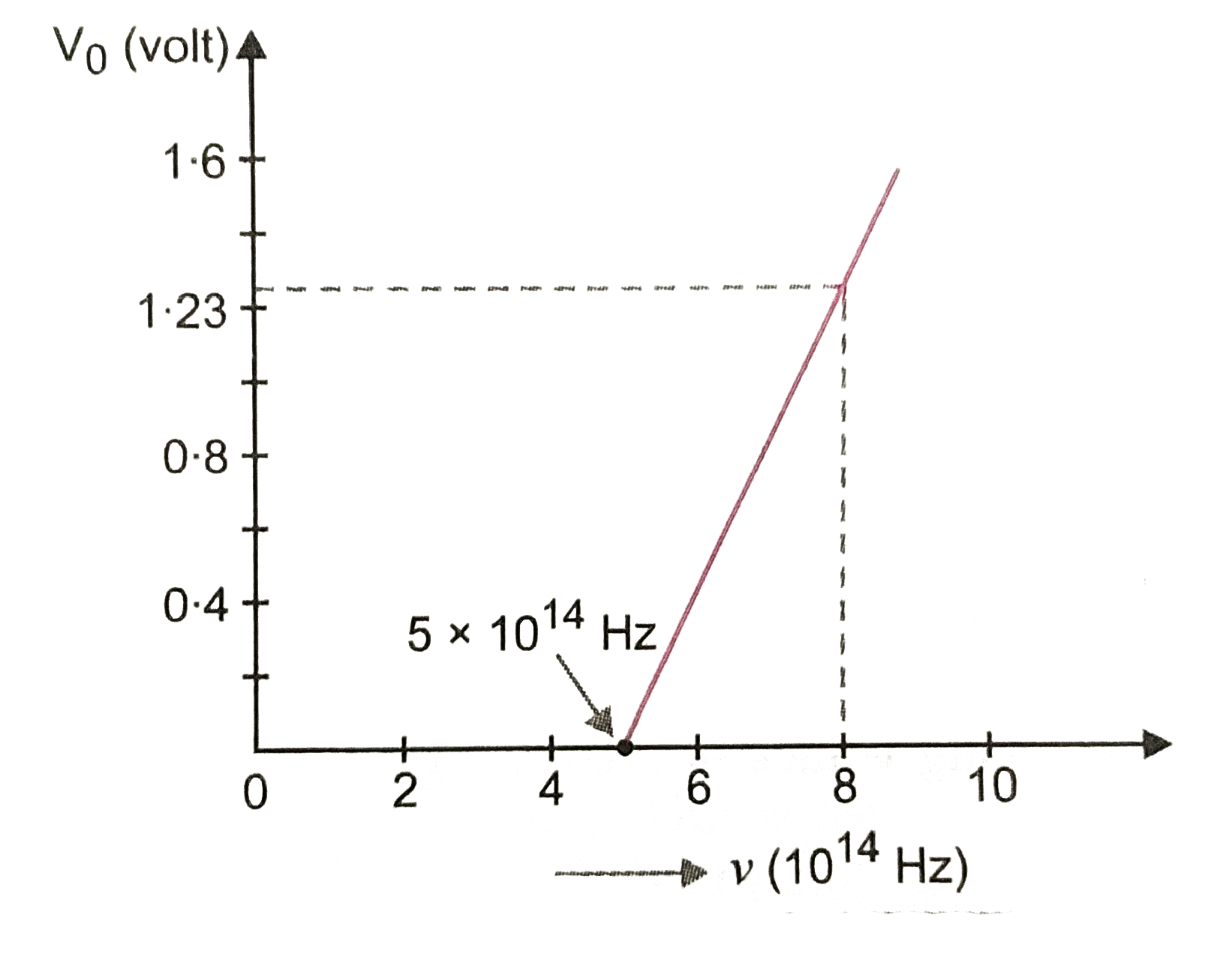
Using the graph shown in fig for stopping potential vs the incident frequency of photons, calculate Planck's constant.

Planck's constant (h), speed of light in vacuum (c) and Newton's gravitational constant (G) are three fundamental constants. Which of the following combinations of these has the dimension of length?
![Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ] Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ]](https://dwes9vv9u0550.cloudfront.net/images/8714373/032957da-34c2-47ec-a991-027613566c64.jpg)
Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ]
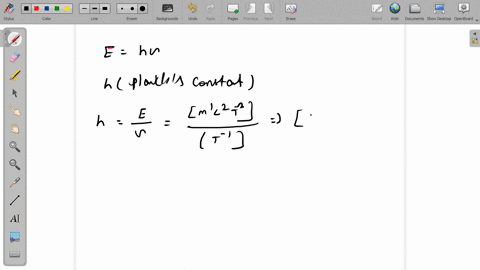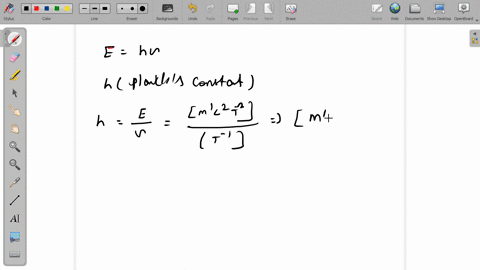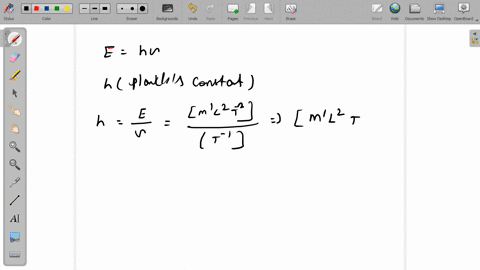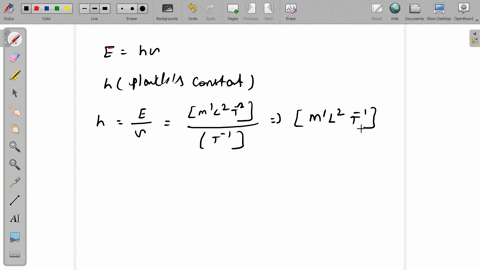
SOLVED:The dimensions of Planck's constant are the same as that of (a) linear impulse (b) work (c) linear momentum (d) angular momentum